INTRODUCTION
Olive flounder (Paralichthys olivaceus) is an economically important fish species in East Asia including in Korea, contributing about half of the economic value of domestic aquaculture production (KOSIS, 2018). However, the survival rate of olive flounder is seriously affected by the aging of aquafarms, natural disasters, rapid temperature changes, high density rearing, and emergence of infectious diseases (Kim et al., 2003, 2009). Especially, a viral hemorrhagic septicemia virus (VHSV) is highly pathogenic, causing severe economic losses owing to the high mortality rate of infected fish. Nevertheless, an effective therapeutic agent is not known.
Fishes infected with VHSV have enlarged kidneys and spleens, protruding eyes, and hemorrhagic fins and gills (Kim et al., 2009; Duesund et al., 2010). The disease has been reported worldwide in freshwater fishes and marine fishes. In Korea, the disease occurs between winter and spring, during the period of low water temperature, causing serious damage to the olive flounder aquaculture industry (Kim et al., 2003; OIE, 2018).
The survival of fish in the aquatic environment starting from the fry stage is strongly dependent on the innate immune system (Beck & Habicht, 1996; Fischer et al., 2013). Interleukin-8 (IL-8; also known as CXCL8, NAF-1, MDNCF-1, and NAP-1) is a typical chemokine belonging to the CXC-subfamily of cytokines involved in innate immunity (Fernandez & Lolis, 2002; Esche et al., 2005). IL-8 is a heparin-binding protein comprised of a three stranded β-sheet and an α-helix. It is an inflammatory cytokine produced by various cells, including macrophages, when inflammation is induced, and it activates inflammatory cells by the synergistic action of NFkB, C/EBP, and AP-1 binding elements in the inflammatory reaction (Zlotnik & Yoshie, 2012). During an acute inflammatory reaction, it also acts as a neutrophil leukocyte chemoattractant of activators, causing various diseases (Kapp et al., 1994; Taub et al., 1996; Sun et al., 2011).
In the olive flounder, the nucleotide sequence of IL-8 was described by Lee et al. (2001) and subsequently, several studies were conducted (Zhao et al., 2015; Guo et al., 2018). IL-8 has also been reported in channel catfish (Ictalurus punctatus), common carp (Cyprinus carpio), haddock (Melanogrammus aeglefinus), large yellow croakers (Larimichthys crocea), rainbow trout (Oncorhynchus mykiss) and others, but information on the immune response is limited (Laing et al., 2002; Huising et al., 2003; Chen et al., 2005; Miyar et al., 2007; Nor et al., 2008; Zhou et al., 2018; Wang et al., 2019a,b; Xiao et al., 2019). Therefore, in this study, we analyzed the expression of IL-8 at the early developmental stage and in tissues of the olive flounder in order to investigate the immune mechanisms of IL-8. We also examined its expression in various tissues after VHSV infection.
MATERIALS AND METHODS
Olive flounders used in the experiments were grown in a 3-ton flow-through tank at 19±1°C with natural photoperiod at the Breed Research Center of the National Fisheries Research and Development Institute. To observe gene expression at different developmental stages, samples were collected until 18 days after hatching, placed in TRI-Solution™ (Bio Science Technology, Daegu, Korea), and stored at −80°C until RNA isolation. To analyze gene expression in different tissues, tissue samples were collected from the brain, eye, fin, gill, intestine, kidney, liver, muscle, spleen, and stomach from healthy 8-month-old olive flounders (total length approximately 30 cm) and stored at −80°C until testing. The stress during sampling for each group was minimized by anesthesia using 150 ppm MS-222 (Sigma-Aldrich, St. Louis, MO, USA) (Noh et al., 2017).
The VHSV challenge experiment was conducted on selected olive flounders (body length of approximately 30 cm, 8 months old) which were divided into control group and VHSV-infected group. The control group was injected with 100 μL of phosphate buffered saline, and the test group was injected a VHSV suspension (104.8 TCID50 virus/fish) (Kong et al., 2009). Each group was maintained at 13°C in a recirculation system, without flow and feeding. Samples from the fin, gill, kidney, liver, muscle, and spleen were collected 0, 3, 6, 12, 24, 48, 72, and 96 h after inoculation. Each sample was pooled in equal amounts and frozen in liquid nitrogen. Frozen tissues were ground using a homogenizer and subjected to RNA extraction. The stress for each group was minimized by anesthesia using 150 ppm MS-222 (Sigma, USA) (Noh et al., 2017).
Total RNA was extracted from each sample using the modified protocol with TRI-Solution™ (TS200-001; Bio Science Technology, Korea). The sample (0.2 g) was homogenized with 1 mL of TRI-Solution and allowed to react at room temperature for 5 min. Then, 0.2 mL of chloroform was added, inverted for 15 s, and centrifuged at 13,400 ×g and 4°C for 15 min. The same volume of isopropyl alcohol was added to the separated supernatant, and the mixture was inverted for 15 s and centrifuged for 10 min at 13,400 ×g and 4℃. The supernatant was decanted, rinsed with 75% ethanol and completely air dried. The air-dried sample was dissolved in sterile distilled water and treated with DNase-I (Sigma-Aldrich, USA) to remove gDNA contamination. Total RNA was evaluated using spectrophotometry (Eon™ Microplate Spectrophotometer; BioTek, Winooski, VT, USA) by measuring the absorbance at A260/280 and A260/230, and the samples were stored at −80°C until further use. The isolated RNA was converted into complementary DNA (cDNA) using the Transcriptor First Strand cDNA Synthesis Kit (Roche Ltd., Mannheim, Germany) according to the manufacturer’s protocol.
Gene expression patterns of IL-8 were analyzed using quantitative real-time PCR (qRT-PCR) (Applied Biosystems 7500 Fast Real-Time PCR System; Applied Biosystems, Foster City, CA, USA). qRT-PCR was performed using 100 ng/μL cDNA samples in Fast SYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) under the following conditions: an initial denaturation step for 20 s at 95°C, 40 cycles of 3 s at 95°C and for 30 s at 60°C, followed by a final dissociation stage. IL-8 and 18S rRNA primers (GenBank accession numbers AF216646.1 and EF126037.1, respectively) were used for the analysis. The nucleotide sequences of IL-8 and the housekeeping gene 18S rRNA was obtained from the GenBank database. The IL-8 and 18S rRNA primers of the olive flounder were designed using the Primer3 program (Rozen & Skaletsky, 2000). The primers used for the analysis were as follows: IL-8 forward (5′-GTTGTTGCTGTGATGGTGCT-3′) and IL-8 reverse (5′-GCCGGTATCTTTCAGAGTGG-3′), 18S forward (5′-ATGGCCGTTCTTAGTTGGTG-3′) and 18S reverse (5′-CACACGTGATCCAGTCAGT-3′). Standardization of the total cDNA amount was performed using the 2−ΔΔCt method and 18S rRNA of the olive flounder as an internal standard for data normalization (Pfaffl, 2001).
All sampling was performed in three replicates to ensure the accuracy of experimental findings, and all data are expressed as mean±SE (n=3). The software R 3.0.1 (R Development Core Team, 2013) was used for statistical analysis of the results obtained in this study. Significant differences in data values were confirmed by one-way analysis of variance (ANOVA). Significance test for gene expression in the individuals was performed using the Tukey’s honestly significant difference test at p<0.05.
RESULTS
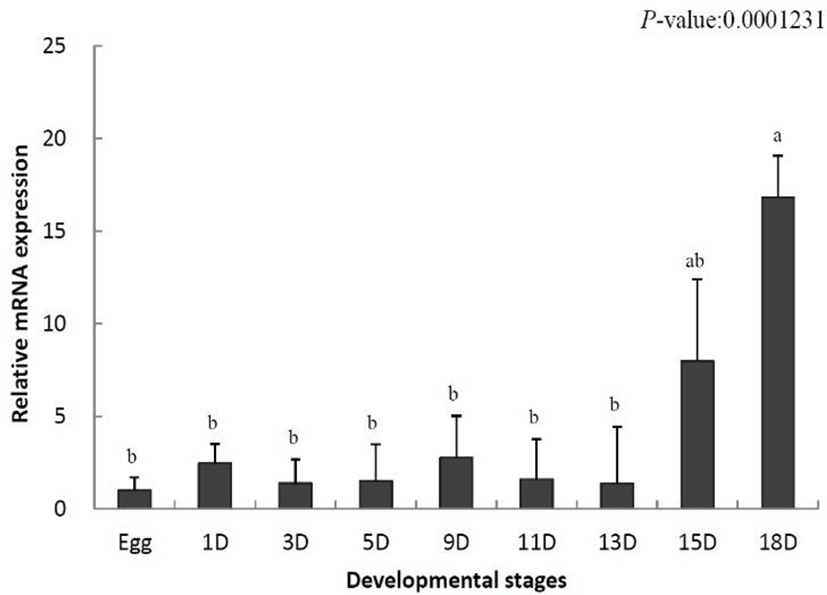
To identify the role of IL-8 in the early developmental stage, the temporal expression was analyzed using samples from eggs to 18-day-old larvae by qRT-PCR. The data were standardized to the values obtained for egg (set to 1.0) of the olive flounder. IL-8 mRNA levels were increased on day 1 after hatching (2.4-fold) and began to decrease from day 3 (1.3-fold) (Fig. 1). Thereafter, the decrease and increase were repeated. IL-8 mRNA levels increased significantly on day 15 (7.9-fold) and reached the highest level on day 18 (16.8-fold) (Fig. 1). In conclusion, IL-8 was not expressed in the early developmental stages, but its levels increased in later stages.
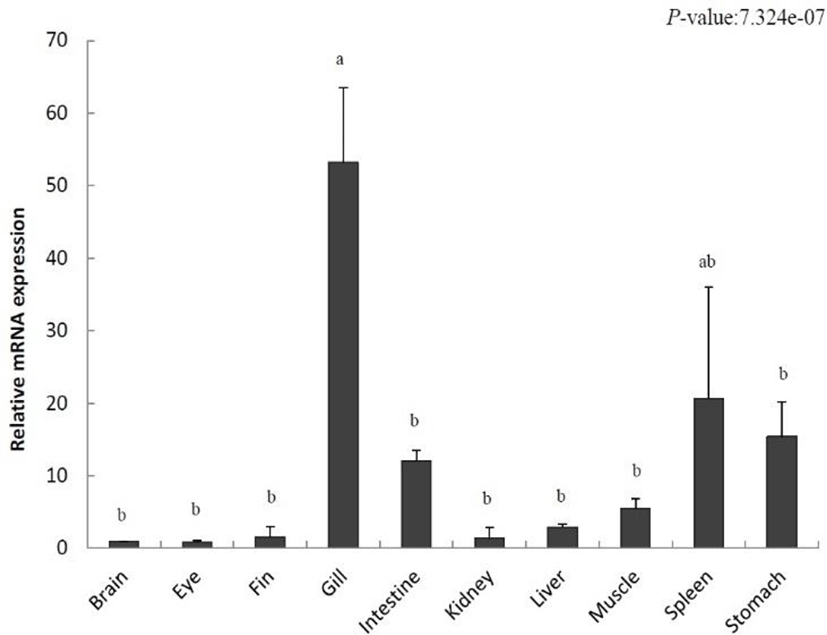
To localize IL-8 in various tissues from the olive flounder and identify its function, we evaluated the expression of IL-8 using qRT-PCR. Relative tissue-specific gene expression was investigated by comparing the results with the expression of IL-8 in the brain tissue (the value set to 1). IL-8 was increased in the fins (1.5-fold), gills (53.1-fold), intestine (12.0-fold), kidney (1.3-fold), liver (2.9-fold), muscle (5.5-fold), spleen (20.6-fold), and stomach (15.4-fold) (Fig. 2). The highest gene expression of IL-8 was detected in the gill and it was relatively low in the eye. Our results show a differential expression of IL-8 in various tissues.
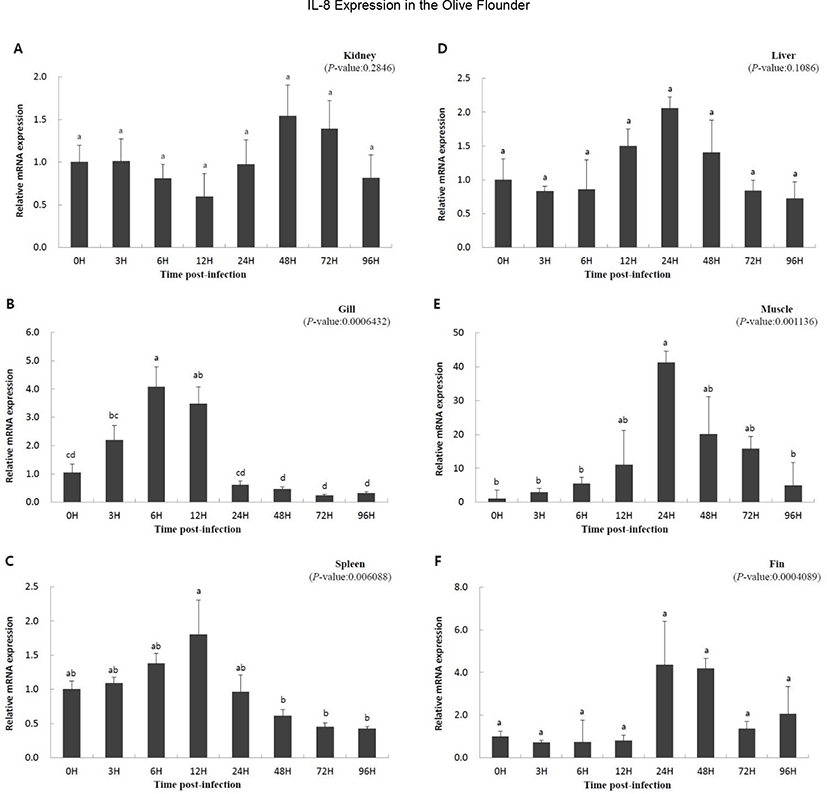
To study the function of IL-8 in the immune response against viral infection, we investigated the expression of the gene using qRT-PCR over a period of time in olive flounder infected with VHSV. Relative gene expression was compared with that at 0 h, which was the starting point of the artificial infection. Overall, a similar pattern of expression was observed. The expression was slow or not significant until a specific time, but increased with time, and then decreased. The expression level in the kidney increased at 48 h (1.542-fold) and then decreased (Fig. 3A); IL-8 expression in the liver increased from 12 h (1.499-fold) to 24 h (2.054-fold) and then decreased (Fig. 3D). The muscle and gill showed increasing patterns of gene expression from the beginning (Fig. 3B and E). In the muscle, IL-8 expression reached highest level at 24 h (41.223-fold) and then decreased rapidly (Fig. 3E). The gill also showed high expression of the gene until 12 h (3.472-fold), which was followed by a rapid decrease after 24 h (0.610-fold) (Fig. 3B). The expression levels in the fin and spleen did not show significant differences early on; the expression in the fins increased from 24 h (4.348-fold) to 48 h (4.184-fold) and then decreased (Fig. 3C and F), while the expression in the spleen increased at 12 h (1.805-fold) and then decreased (Fig. 3C). In conclusion, the expression of IL-8 was not continuous since the time of virus inoculation, but it was expressed later and its expression decreased thereafter.
DISCUSSION
IL-8 is a small inflammatory cytokine, produced by many macrophages. It acts as a secondary mediator protein in inflammatory reactions, activating inflammatory cells, and plays an important role in the inflammatory response in animals by acting as a neutrophil leukocyte chemoattractant or activator (Laing et al., 2002; Laing & Secombes, 2004; Wang et al., 2019b). Accordingly, in this study, the properties of IL-8 were investigated in the olive flounder infected with VHSV.
During the development of the olive flounder, the membranous fin develops immediately after hatching and the gills are formed on day 6. The digestive tract is differentiated on day 10, and completely develops by day 14 (Kim, 2009). IL-8 was not expressed in the early developmental stages its expression increased after day 15 (Fig. 1). Based on these results, IL-8 is not involved in the early stages of development and the fully formed immune system is present only in later stages of the development.
IL-8 was expressed in various tissues of the unchallenged olive flounder. In particular, its expression was the highest in gills, the respiratory organs responsible for the control of gas emission and osmotic pressure but also the most exposed organs to the aquatic environment and the route of invasion of many microorganisms (Fig. 2). This result is consistent with that found in rainbow trout, tongue sole (Cynoglossus semilaevis), and common carp (Laing et al., 2002; Mark et al., 2003; Sun et al., 2011). In addition, high IL-8 expression levels in fishes such as ayu (Plecoglossus altivelis), cobia (Rachycentron canadum), rainbow trout, and tongue sole were detected in the spleen, an important immunological organ (Laing et al., 2002; Sun et al., 2011; Chu et al., 2014; Nguyen et al., 2017). In contrast, a high expression level was observed in the liver and kidneys of the Siberian sturgeon, yellow croaker, and cobia. Our results showed that IL-8 expression occurs predominantly in immune-related organs under normal physiological conditions.
It has been reported that IL-8 expression is increased by the onset of a disease, suggesting an important role of fish IL-8 in antiviral immune response (Chu et al., 2014; Mu et al., 2015; Wang et al., 2019). In the present study, IL-8 expression was significantly increased in the fin, gill, muscle, and spleen by VHSV infection, suggesting that IL-8 is involved in the immune response of the olive flounder infected with VHSV (Fig. 3B, C, E, and F). Similar to our results, induction of IL-8 in the liver, spleen, kidney, and gill by disease infection was observed in cobia, channel catfish, blue catfish, tongue sole, ayu, and the large yellow croaker (Chen et al., 2005; Tian et al., 2010; Sun et al., 2011; Chu et al., 2014; Mu et al., 2015; Nguyen et al., 2017). However, the expression of IL-8 in the kidney and liver was not significantly different between infected olive flounder individuals and the control and did not corroborate previous studies, suggesting that the expression of IL-8 in the kidney and liver could be variably regulated in other fish species (Fig. 3A, D).
In this study, we investigated the immune response of IL-8, an inflammatory cytokine, in the activation of inflammatory cells in the olive flounder. The expression of IL-8 in a developing olive flounder was detected when the immune system of the olive flounder was formed. Its expression was high in the gills and spleen, the organs associated with immunity and metabolism. Infection with VHSV increased IL-8 expression in the fin, gill, muscle, and spleen. These results suggest that IL-8 plays an important role in the innate immune system of fish and will be useful as a basis for studying the immune response of fish.







