INTRODUCTION
Temperature is one of common variables in the aquatic environment that directly affect survival of marine organisms. The ability to tolerate various temperature ranges differs with species (Kindle & Whitmore, 1986). Under culture conditions, fluctuations in water temperature affect the enzyme reaction, growth efficiency, reproduction and immune ability in fish and sudden fluctuation in water temperature that exceed the threshold values often cause deaths of fish (Chatterjee et al., 2004; Cheng et al., 2013). Temperature also changes the physiological functions associated with the stress response of fish. The physiological stress response in fish firstly includes the release of stress hormones such as cortisol. An increase in plasma cortisol levels of fish is generally followed by an elevation in plasma glucose levels. Biochemical parameters, such as cortisol and glucose levels in plasma can be used as general stress indicators in fish (Santos & Pacheco, 1996). In addition, hematological parameters included hematocrit and hemoglobin are good indicator to assess fish health management in various conditions such as exposure of stress (Chen et al., 2004).
The red spotted grouper, Epinephelus akaara is a serranid fish distributed mainly in southern Japan, Korea, China and economically important species in aquaculture industry. Broodstock management and larval rearing techniques of red spotted grouper have been studied for aquaculture of this species.
Changes in the hematological and biochemical responses to water temperature shock have been studied in goldfish, rainbow trout, tilapia, atlantic cod, silver catfish and olive flounder (Basu et al., 2001; Lermen et al., 2004; Yang & Yeo, 2004; Gollock et al., 2006; Hur & Habibi, 2007; Jeong et al., 2012). However, none of the studies explored the physiological stress responses to temperature shock in red spotted grouper.
Therefore, we investigated the hematological and bio-chemical responses in red spotted grouper during and after exposure to water temperature shock. This study may help in promoting suitable grouper culture management in aquaculture.
MATERIALS AND METHODS
Red spotted grouper ranging in weight from 47.6 to 90.8 g were obtained from a private farm (Muangun, Jeolla-namdo, Korea) in winter season. The fish were shipped to the laboratory and acclimated in glass aquaria (80 × 60 × 40 cm) with running seawater of 34 ± 0.5 ‰ at water temperature of 15.0 ± 1.0°C for 3 weeks. The water temperature of the rearing facility was increased from 15°C to 25°C stepwise at rate of 1°C h–1 and kept at 15, 20 and 25°C for experimental periods (7 days). During the accli-mation period, the fish were fed artificial feed twice a day, but were not feed 24h prior to the experiment. During the experimental period, the salinity, dissolved oxygen, and pH were maintained at 34 ± 0.5 ‰, 6.5 ± 1.0 mg l–1, and 7.8 ± 0.3, respectively.
For analysis of the hematological and biochemical responses, fish (n=3 fish/group) were randomly collected from each exposure group (15 as control, 20 and 25°C) at 2 and 7 days and anesthetized in 0.1% 2-phenoxyethanol in seawater. The blood was immediately collected from caudal artery with a heparinized 2 mL syringe.
Hematological indices were determined such as hematocrit (Ht) values and hemoglobin (Hb) levels. Ht values were determined after blood sampling using glass capillary tubes and centrifuged for 5 min at 12,000 rpm in a micro-hematocrit centrifuge (HAWKSLEY AND SONS Ltd., England). Hematocrit readings were performed with micro-hematocrit reader (HAWKSLEY AND SONS, England). Hb levels were measured by cyanomethemoglobin method according to Lee et al. (1998).
Collected blood was separated by centrifugation (15 min at 13,000 rpm) at 4°C and stored at –80°C until analysis. Glutamic oxaloacetic transaminase (GOT), glucose levels in plasma were assessed using an automatic analyser (FUJI DRI-CHEM 4000i, Fujifilm Co., Japan). The automatic analyser was operated using select testing slides (multi-layered slides, Fujifilm Co., Japan) by reading the bar codes via lasers.
For measurement of cortisol level in plasma, steroids from plasma were extracted twice in 2 mL diethyl ether, dried under nitrogen gas and resuspended in phosphate buffer (pH=7.5). Plasma cortisol level was measured by radioimmunoassay (RIA) according to the Kobayashi’s method (Kobayashi & Mikuni, 1987). Antiserum for cortisol was purchased from Cosmo-Bio Co. Ltd. (Tokyo, Japan). Non-radioactive steroid standards was purchased from Steraloids Inc. (Wilton, NH, USA). Radio-labeled steroids ([3H]-cortisol) was purchased from Amersham Lifesciences (Piscataway, NJ, USA).
RESULTS
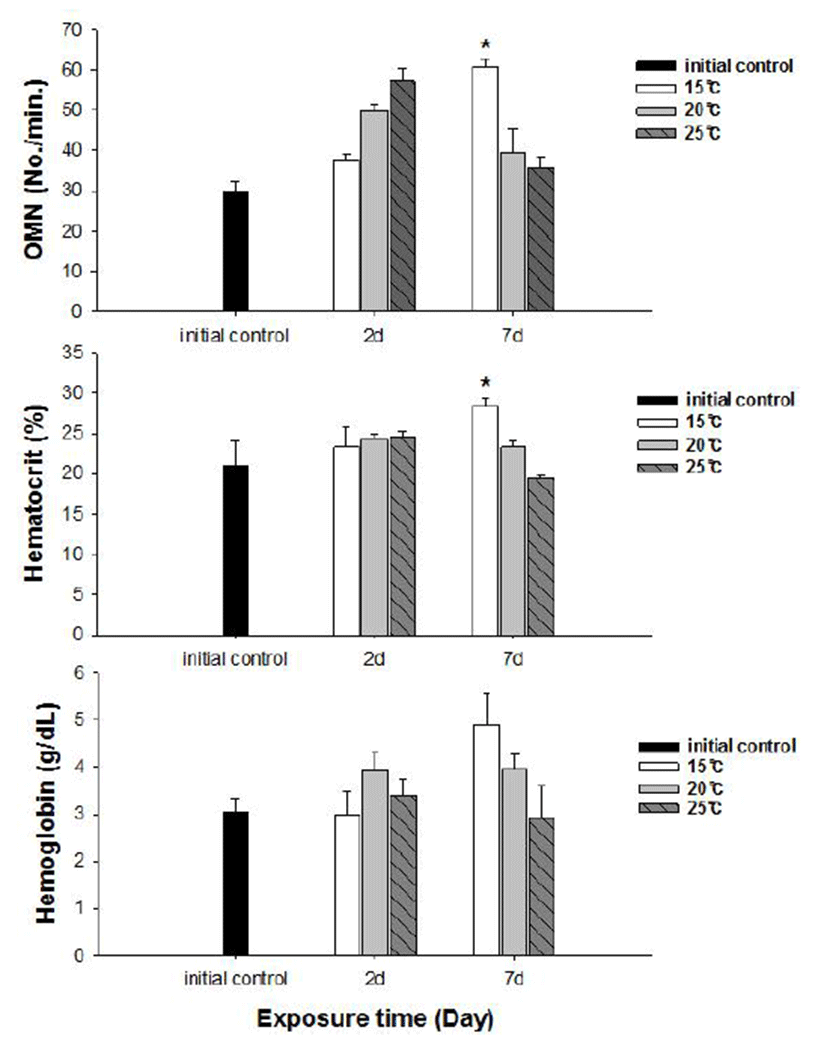
No fish mortality was observed during the experimental period. Results of hematological and immunological activities analysis of red spotted grouper exposed to water temperature are presented in Fig. 1, 2 and 3, respectively. OMN of fish exposed to 15°C lowest among temperature treatment groups at 2 days after exposure (DAE) but showed significantly increase at 7 DAE (P<0.05; Fig. 1). In hematological indices, both Ht and Hb had no differences among fish from temperature groups at 2 DAE. However, Ht and Hb values changed within each treatment groups at 7 DAE. Red spotted grouper exposed to 15°C had significantly higher Ht value at 7 DAE (P<0.05; Fig. 1). In biochemical indices, plasma glucose levels within each experimental group were similar to the hematological responses. At 7 DAE, plasma glucose levels had apparent elevation at 15°C. No differences in plasma cortisol levels were found within each treatment throughout the experiment period (P<0.05; Fig. 2) Also, GOT level in plasma was significantly increased at water temperature of 15°C (P<0.05; Fig. 3). During the experimental period, the correlation between water temperatures with all parameters tested in this experiment was statistically tested by analyzing the data obtained during the water temperature exposure. Only OMN, glucose, Ht and GOT levels showed significant correlation (P<0.05) with water temperature of 15°C that these parameters confirmed positive correlation.


DISCUSSION
Most groupers are considered to be commercially important marine fish in southeastern Asia. In particular, the red spotted grouper (Epinephelus akaara) is a highly priced market values fish in this region (Rimmer, 2004; Lee et al., 2014). Groupers have been reared are warm water fish that spawn and grow best at 24 to 30°C; most of them can tolerate a range of 15 to at least 35°C (Tucker, 1999). The present study was performed to evaluate the impact of different temperature range (15°C, 20°C, and 25°C) on the growing of red spotted grouper during the winter season. For the control group, temperature was kept at 15°C that is over-wintering temperature.
We determined the opercula activity, hematocrit, hemo-globin, glucose, cortisol, and GOT levels at the end of experiment. During the experiment period (7 days), OMN, hematocrit (Ht), glucose and GOT values were significantly high in 15°C when compared to 20 and 25°C. Hemoglobin value was also increased at 15°C, but no significant differences.
Cortisol and glucose levels are general stress indicators in fish (Pacheco & Santos, 2001). In this study, plasma glucose values were not correlated to the observed plasma cortisol response. There was no differences in cortisol levels among the temperature groups. Chen et al. (1995) reported that plasma cortisol concentrations increased in common carp only after acute exposure to 4°C, but in the chronic experiment, they returned to levels similar to those of the control group. The results obtained with silver catfish (Rhamdia quelen) showed that plasma cortisol levels were unchanged in both the acute and chronic treat-ments (Lermen et al., 2004). High blood glucose levels at low temperatures is indicative of retarded metabolism, and is also an index of sub-lethal stress (Best et al., 2001). In our results, 15°C is likely more stressful to red spotted grouper than 20°C and 25°C during the winter. Moreover, glucose was a better index than the cortisol in this fish. In addition, a significantly higher value of GOT activity was recorded in 15°C. This coincides with the increase in value of glucose which is related to a decrease in glycogen reserves in the liver (Click & Engin, 2005). Under cold conditions, the silver catfish spared glucose, resulting in gluconeogenesis, whereas in the warm water, it likely consumes glucose (Lermen et al., 2004). Tandon & Joshi (1974) reported that serum glucose level increased with the fall of temperature in Clarias batrachus. They observed the highest blood glucose level during the winter and lowest during the summer and correlated it with the lowering of temperature.
Hematological parameters such as Ht and Hb are used to assess the functional status of the oxygen carrying capacity of the blood stream (Shah & Altindag, 2004) and assess the physiological status of fish under the stressed condition (Fernandez & Mazon, 2003). Opercular beat rate also has been used to provide a measure of response to stress in fishes. Counting operculum movement is a way to calculate respiration rates. Increased opercular activity can lead to increased oxygen consumption (Dalla Valle et al., 2003). Gibson & Mathis (2006) explained that opercular beat rate as an assay for thermal stress alone is limited due to the responsiveness in a variety of contexts. This behavior, however, appears to be another reliable response in predator recognition, and could be preparatory to offensive or defensive movements, or exploratory behavior (Rottmann et al., 1992). Our results showed increase in both Ht and OMN at 15°C temperature. These observations along with the results of glucose level in plasma confirm that red spotted grouper adapts better to temperatures between 20 and 25°C during the winter season.







