INTRODUCTION
The adult body of most bilaterians shows stereotypic left-right asymmetry. In vertebrates, the internal organs are not symmetric, as the placement of the heart and liver is determined along the left-right axis, and there are different structures in the left and right regions of the brain (Hamada et al., 2002; Levin, 2005). In the early stages of vertebrate embryonic development, the morphology is bilaterally symmetrical, but later the symmetry becomes broken along the left-right axis. The decisive event in left-right axis formation is the expression of Nodal gene on the left side of the embryos (Boorman & Shimeld, 2002; Hirokawa et al., 2006; Schweickert et al., 2017). The important thing among them is that the Nodal gene appears to be expressed in the lateral plate mesoderm. The mechanisms for expressing the Nodal gene only on the left side of the embryo differs among vertebrates. It was suggested that the liquid flow produced by motile cilia in the node may be responsible for left-right axis formation (Okada & Hirokawa, 2009; Vandenberg & Levin, 2013; Blum et al., 2014). One of the essential genes activated by Nodal appears to encode the transcription factor Pitx2, which normally is expressed only on the left side of vertebrate embryos. It is likely that Nodal signaling establishes left-right polarity by activating Pitx2 on the left side is conserved throughout all vertebrates (Blum et al., 2014; Little & Norris, 2021).
Ascidians are phylogenetically the closest relatives of vertebrates. The ascidian larvae, which display a chordate ground body plan, are left-right asymmetric in several structures, including the brain vesicle (Hirano & Nishida, 2000; Boorman & Shimeld, 2002; Ryan et al., 2016). The only two melanized pigment cells of ascidian larvae exist in the brain vesicle and they belong to two different sensory organs, ocellus and otolith. The ocellus has a cup-shaped pigment cell containing numerous small melanin granules, while the otolith contains a single large melanized pigment cell attached to the ventral surface of the brain vesicle (Nishida & Satoh, 1989; Nicol & Meinertzhagen, 1991). The ocellus and otolith are part of the organ involved in sensing light and gravity, respectively. The ocellus is located on the right side of the brain vesicle. However, the otolith is present near the center of the brain vesicle but exists slightly to the right. The coronet cells, which have been speculated to be components of a sensory system, are asymmetrically located on the left side of the brain vesicle (Nicol & Meinertzhagen, 1991; Taniguchi & Nishida, 2004). The coronet cells are dopaminergic, but their function is not yet known (Moret et al., 2005). The coronet cells were received their name from the coronet cells of the saccus vasculosus, an organ that serves as a photoperiodic and seasonal sensor in teleosts (Nakane et al., 2013; Kourakis et al., 2021).
Parkin co-regulated gene (PACRG) was first reported as a gene closely related to the Parkinson’s disease associated gene Parkin (PARK2) in humans (West et al., 2003). PACRG is oriented in a head-to-head array with the Parkin gene on human chromosome 6. Expression of the PACRG and Parkin genes appears to be regulated by a shared bi-directional promoter (Imai et al., 2003; West et al., 2003; Stephenson et al., 2018). It is likely that Parkin and PACRG encoded proteins interact and function in the same biological pathways. Parkin encodes an RBR E3 ubiquitin ligase involved in tagging defective proteins for degradation by proteasome (Shimura et al., 2001; Spratt et al., 2014). The exact function of the PACRG is not well understood, but it is implicated in protecting cells against protein aggregation, potentially by a mechanism related to that of the Parkin. PACRG is an evolutionarily very highly conserved gene, which is present from green algae to mammals (West et al., 2003; Dawe et al., 2005; Ikeda et al., 2007). During early embryogenesis, PACRG expression is specifically localized to epithelia where leftward flow arises, that is, Kupffer’s vesicle in zebrafish, the gastrocoel roof plate in Xenopus and the posterior notochord in mammals (Thumberger et al., 2012). The expression of PACRG is observed in various tissues of adult mice, and it is particularly prominent in regional brain area of newborn mice such as the ependymal cells and cilia lining the ventricles (Brody et al., 2008; Wilson et al., 2009). In human, PACRG is expressed in astrocytes throughout the brain and in pigmented noradrenergic neurons of the locus coeruleus (Taylor et al., 2007). In Xenopus, PACRG-specific signals are detected in multiciliated choroid plexus cells, thalamic nuclei and in the ventral midline of tadpole larvae (Thumberger et al., 2012). The PACRG-MO (morpholino oligonucleotide) injected Xenopus embryos result dose dependently in left-right asymmetry, neural tube closure and gastrulation defects. These results suggest that PACRG may play an important role in motile cilia formation, and function in development and left-right axis formation of the brain in vertebrates.
In this study, I report an ascidian PACRG orthologue that is specifically expressed on the left side of the brain vesicle. The brain region expressing PACRG appears to include coronet cells known as dopaminergic cells.
MATERIALS AND METHODS
Adults of ascidian Halocynthia roretzi were purchased about a month before the spawning season from fishermen in the vicinity of Institute of Ocean Science Education, Gangneung-Wonju National University. Ascidians were reared in seawater at 9°C under 24-hour lighting. Eggs were spawned under temperature and light control, and they were fertilized with a suspension of non-self sperm. Fertilized eggs were raised at 13°C. Embryos were collected at appropriate stages and fixed for whole-mount in situ hybridization and immunofluorescence staining. Tadpole larvae hatched at about 35 hours after fertilization.
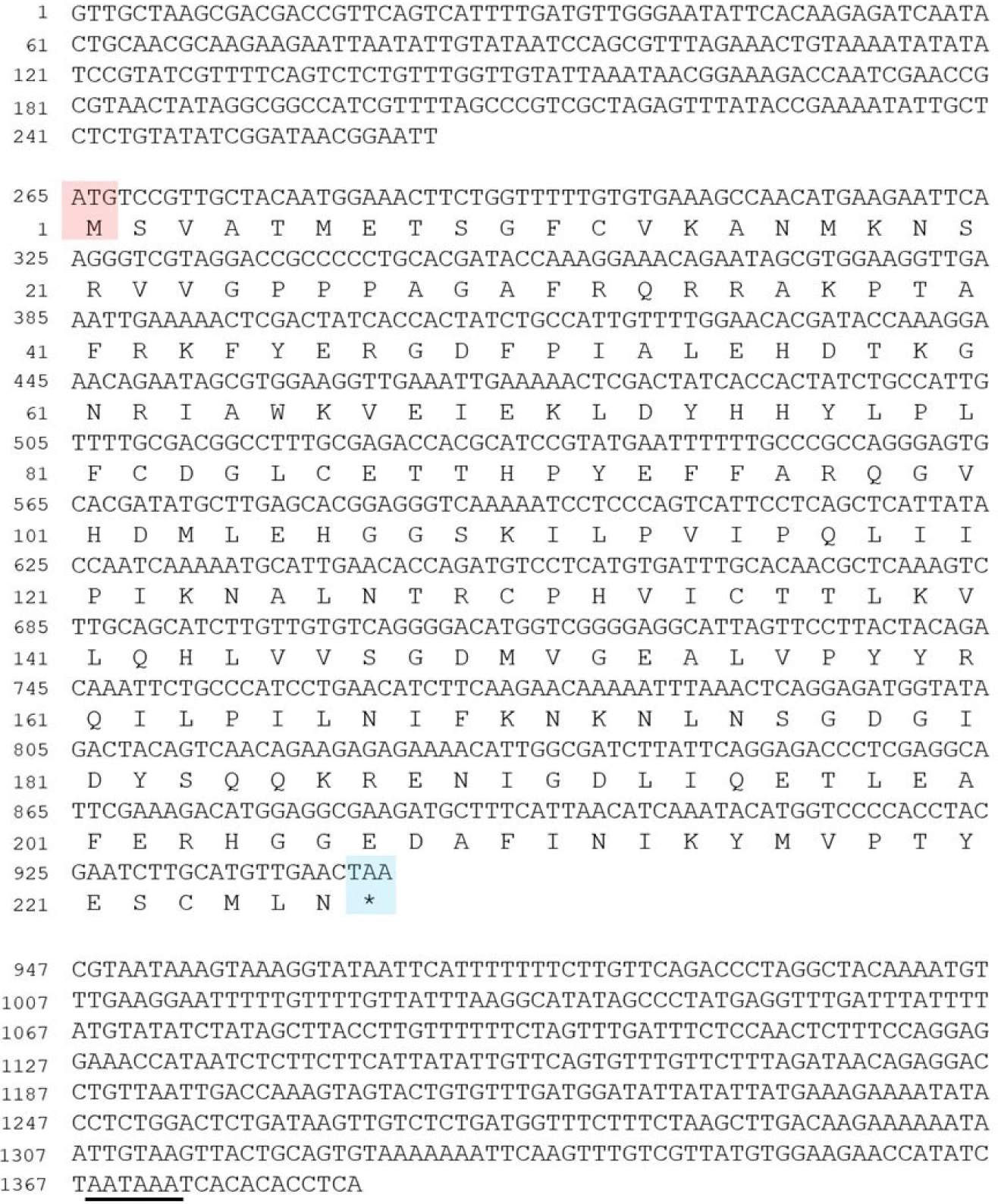
The information (Gene ID: Harore.g00012573) on the PACRG of H. roretzi was obtained from the Aniseed: Ascidian Network for In Situ Expression and Embryological Data (https://www.aniseed.fr; Brozovic et al., 2018). To obtain a full-length cDNA sequence, 5’ RACE and 3’ RACE were performed with the SMART RACE cDNA Amplification Kit (Ambion, Austin, TX, USA). The HalocynthiaPACRG cDNA was obtained, consisting of 1,385 nucleotides and encoding predicted protein of 226 amino acids.
The PACRG probe for in situ hybridization were prepared with a digoxigenin RNA labeling kit (Roche, Basel, Switzerland). The probe was prepared from the full-length PACRG cDNA. In situ hybridization was performed as described by Miya et al. (1997), except that the probes was not hydrolyzed by alkaline treatment.
The Hpr-1 monoclonal antibody specifically stains the coronet cells in Halocynthia late-tailbud embryos and larvae (Darras & Nishida, 2001; Taniguchi & Nishida, 2004). Coronet cells were previously thought to be hydrostatic pressure organ cells (Eakin & Kuda., 1971; Moret et al., 2005). Immunohistochemical staining for coronet cells was carried out by standard methods using a TSA fluorescein system (PerkinElmer Life Sciences, Waltham, MA, USA) according to the manufacturer’s protocol.
RESULTS
To study how left-right asymmetry of the brain vesicle in Halocynthia larva is determined, I attempted to isolate a gene that is expressed in the brain vesicle. As a result, an ascidian PACRG orthologue cDNA, consisting of 1,385 nucleotides and encoding predicted protein of 226 amino acids, was cloned (Fig. 1). The overall degree of amino acid identity between the Halocynthia PACRG and the vertebrate PACRGs is approximately 80%, except for the N-terminus (data not shown; Thumberger et al., 2012). The N-terminus of the Human PACRG protein is about 30 amino acids longer than that of the Halocynthia PACRG protein (data not shown).
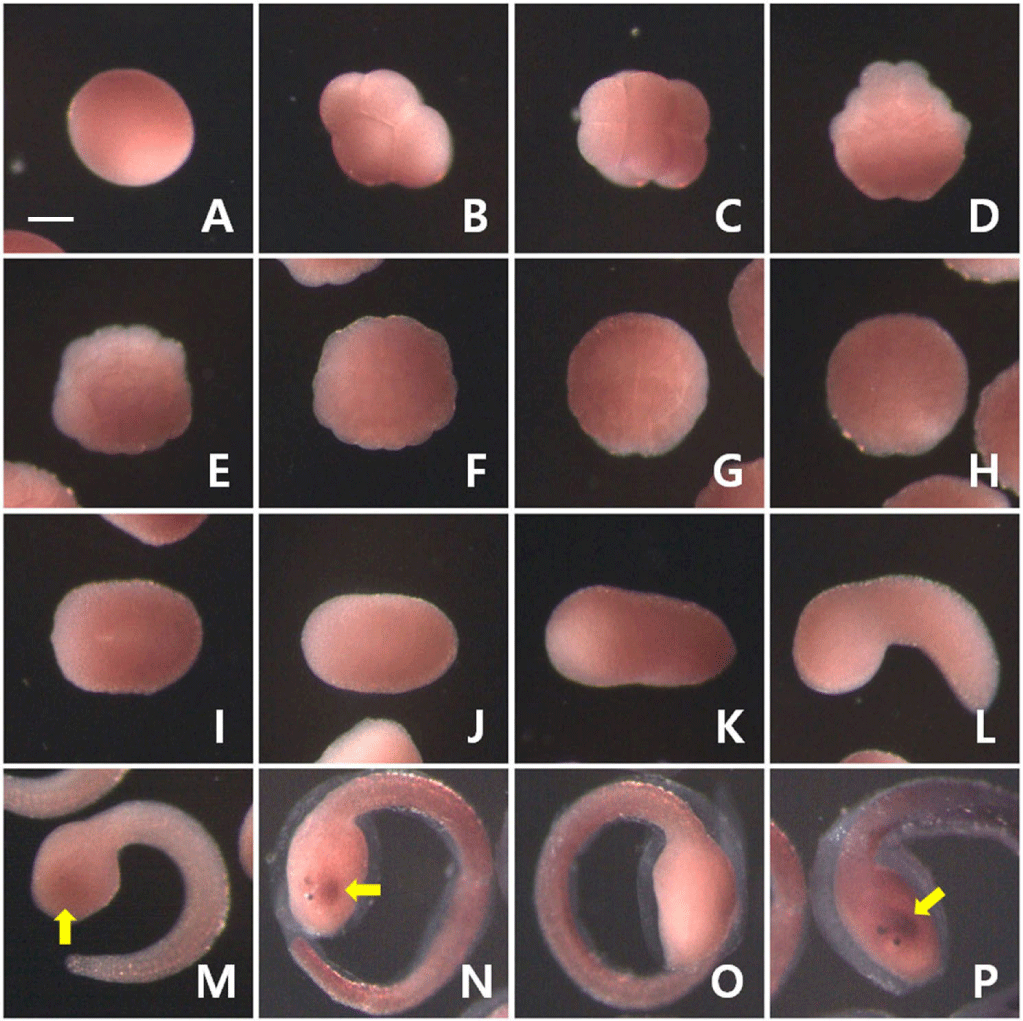
I examined the expression pattern of PACRG mRNA at various developmental stages during Halocynthia embryogenesis using whole-mount in situ hybridization. Maternal expression of PACRG did not observe (Fig. 2). Zygotic expression of PACRG was first detected in the head region at the late tailbud stage (yellow arrow in Fig. 2M). Expression of PACRG appeared to occur only on the left side of the brain vesicle at the just before hatching stage (yellow arrows in Fig. 2N and P). Since the colorimetric reaction took more than 48 hours, it is estimated that the transcripts of PACRG are expressed at very low levels.
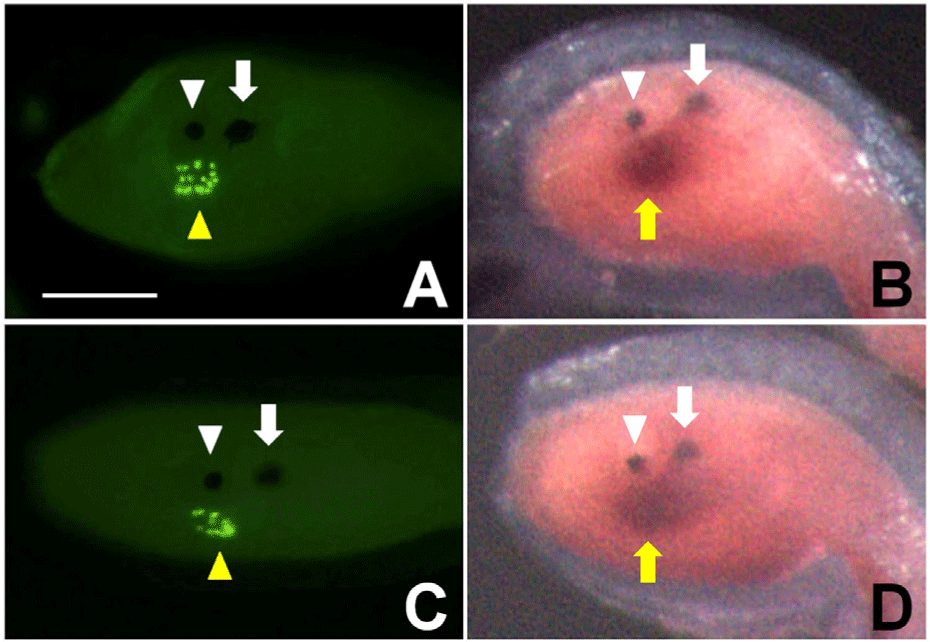
To confirm the location of PACRG expression in the brain vesicle, I compared the staining of coronet cells with the expression of PACRG in the larvae just before or just after hatching (Fig. 3). In ascidians, Ciona and Halocynthia, each coronet cell projects a small globular body into the lumen of the brain vesicle (Eakin & Kuda., 1971; Katz, 1983; Kim et al., 2006). The globular bodies of coronet cells were specifically recognized by the Hpr-1 antibody in Halocynthia larvae (yellow arrowheads in Fig. 3A and C). Coronet cells stained by the Hpr-1 antibody were located on the left side of the brain vesicle (Fig. 3C). This location is thought to be the same as the site of PACRG expression (yellow arrow in Fig. 3D). Therefore, PACRG is presumed to be expressed on the left side of the brain vesicle, including coronet cells, in Halocynthia larva.
DISCUSSION
PACRGs are the evolutionarily very highly conserved genes, which are present in all metazoans, as well as flagellated protozoans (Dawe et al., 2005; Ikeda et al., 2007). PACRG does not have any conserved protein domains that might provide evidence of protein function. PACRG encodes a small size protein that is mostly in an alpha-helical structure. It is thought that the PACRG in Halocynthia would exhibit similar characteristics. PACRGs perform various functions. In Chlamydomonas, PACRG protein plays a role in regulating dynein-driven microtubule sliding in motile cilia (Dymek et al., 2019). PACRG and FAP20 (flagellar-associated protein 20) form the inner junction of axonemal doublet microtubules and regulate ciliary motility. PACRG also localizes to a subset of non-motile cilia in sensory neurons from the nematode Caenorhabditis elegans, where it regulates signaling processes linked to gustatory plasticity (Loucks et al., 2016). It has been reported that direct interactions between MEIG1 (the meiosis-expressed gene 1) and PACRG are essential for the formation of mature sperm cells in mammals (Lehti & Sironen, 2016; Hasse et al., 2023). In elongating spermatids, MEIG1 binds to PACRG to form a temporary complex in the manchette structure that assists with the elongation process by transporting cargo proteins for sperm formation. It is thus necessary to investigate the function of PACRG in ascidians.
PACRG mRNA is expressed in various brain area of vertebrates. The expression of PACRG transcripts is found in specific area of the brain where dopaminergic neurons are abundant, such as the substantia nigra in various vertebrates (Brody et al., 2008; Wilson et al., 2009; Thumberger et al., 2012; Stephenson et al., 2018). In the ascidian Halocynthia larva, PACRG is expressed on the left side of the brain vesicle, which includes coronet cells known as dopaminergic cells. Therefore, it is possible that the PACRG is involved in the development of coronet cells and in the formation of left-right asymmetry of the brain vesicle in Halocynthia larva.
The Nodal and Pitx2 genes are also involved in the formation of left-right asymmetry in ascidians. It has been reported that Nodal and Pitx2 are expressed on the left side of the ascidian embryos at the neurula stage, although the expression is restricted to the epidermis and is not evident in the mesoderm, unlike in vertebrates (Morokuma et al., 2002; Shimeld & Levin, 2006). There is no structure within ascidian embryos in which liquid flow is made by ciliary movements. Moreover, although cilia are present prior to molecular asymmetries, they are not motile in the ascidian Ciona embryos (Thompson et al., 2012). Ascidian embryos rotate along the anterior-posterior axis at the neurula stage, known as neurula rotation (Nishide et al., 2012; Yamada et al., 2019). After the rotation, contact between the left-side epidermal cells and the inner vitelline (chorionic) membrane induces Nodal expression in the left-side epidermis. It appears that an unknown chemical signal(s) originated from the vitelline membrane are involved in the Nodal expression. The ascidian larval brain vesicle had lost left-right asymmetry in the absence of Nodal signaling (Nishide et al., 2012; Kourakis et al., 2021). These results suggest that the expression of PACRG on the left side of the brain vesicle in Halocynthia is regulated by Nodal signaling during the neural and tailbud stages.
