INTRODUCTION
The digestive system of fish is a critical organ complex responsible for nutrient digestion and absorption. Understanding its development, particularly the role of the digestive tract in food digestion processes, is essential for comprehending the physiology of larval fish. This knowledge is fundamental for improving larval survival rates, including optimizing the selection and timing of initial food sources during seed production (Han et al., 2007; Kjørsvik et al., 2007).
Previous studies on marine fish have shown that the development of the digestive system plays a critical role in determining the timing of feeding and the selection of food sources (Peña et al., 2003; Mai et al., 2005; Micale et al., 2006). The larval stage is a crucial period in fish farming due to the morphological and functional changes occurring in the digestive tract and associated organ (Govoni et al., 1986). The transitional phase from endogenous to exogenous feeding, as well as the diet shift from live to formulated food, is often associated with high mortality rates (Sifa & Mathias, 1987; Yúfera & Darias, 2007). In grouper seed production, early mortality is mainly caused by a mismatch between the timing of feeding and the development of the digestive system, which adversely affects growth and survival (Martínez-Lagos et al., 2004). Additionally, providing suitable feed based on the developmental stage of the digestive system is crucial for preventing mass mortality during the transition to the juvenile stage (Tanaka, 1971).
The red spotted grouper (Epinephelus akaara) is one of the grouper species with high economic value in Asia-Pacific countries. Although larval rearing techniques in grouper aquaculture have progressively improved in recent years, challenges in larval production persist. The limited success in intensive grouper rearing is largely attributed to insufficient knowledge of nutritional requirements, as well as the morphological and functional development of the larval digestive tract. Therefore, the purpose of this study was to examine the histological development of the digestive tract in hatchery-reared red spotted grouper from hatching to the juvenile stage, providing foundational knowledge to optimize feeding strategies for larval rearing.
MATERIALS AND METHODS
The eggs of E. akaara used in this study were obtained through induced spawning from broodstock at the Kidang Marine Science Institute, Jeju National University. The eggs were incubated in a circular tank (Ø4.0×1.2 m, effective water volume of 12 tons) at a water temperature of 21±0.1°C, with a stocking density of 20 eggs per liter. The hatching occurred 24 hours after fertilization. During the rearing period, water temperature, dissolved oxygen and pH were 25.5±0.2°C, 6.5–9.5 mg/L and 7.5–8.4, respectively. Fish were reared under natural photoperiod conditions.
The feeding regime of larvae consisted of rotifers (Brachionus rotundiformis) at a density 10-15 individuals/mL from 3 to 30 day after hatching (DAH). Chlorella was provided as feed for the rotifers, with a final density of 1.5×1010 cells/mL. Newly hatched Artemia nauplii were provided from 20 to 25 DAH and enriched Artemia were provided from 26 to 60 DAH. The commercial microparticulated diet was initially provided in minute amounts starting from 12 DAH, with the feeding volume gradually increasing from 20 DAH.
For histological analyses, 30 normal specimens were randomly sampled from the rearing tank every two days, from hatching to 50 DAH. The total length (TL) of the larvae was measured to the nearest 0.1 mm. Fish were fixed in 10% phosphate-buffered formalin, dehydrated in a graded ethanol series, and embedded in paraffin. Serial sagittal sections (5–6 μm thick) were cut from each block, with a total of 30 sections stained using Hematoxylin-Eosin (H&E) and Periodic Acid-Schiff (PAS) reagents. External morphological changes in the larvae were observed using an optical microscope (OLYMPUS DP73) and a stereomicroscope (Zeiss Microscope Stemi SV 11 Apo Stereoscope).
RESULTS
Newly hatched larvae at 1 DAH measured 2.01±0.18 mm TL, with the yolk occupying most of the body. The fins appeared as membranous structures, and the eyes were translucent white, lacking melanophore deposition (Fig. 1A). The digestive tract was observed as a straight structure along the yolk, and the oil globule was located adjacent to the anus at the posterior end of the yolk (Fig. 1B and C). By 3 DAH, the larvae had grown to 2.25±0.15 mm TL, and melanophore deposition had begun in the eyes (Fig. 1D). The yolk-sac was mostly absorbed and surrounded by developing liver and pancreatic tissues, while the lumen of the digestive tract gradually expanded (Fig. 1E and F).
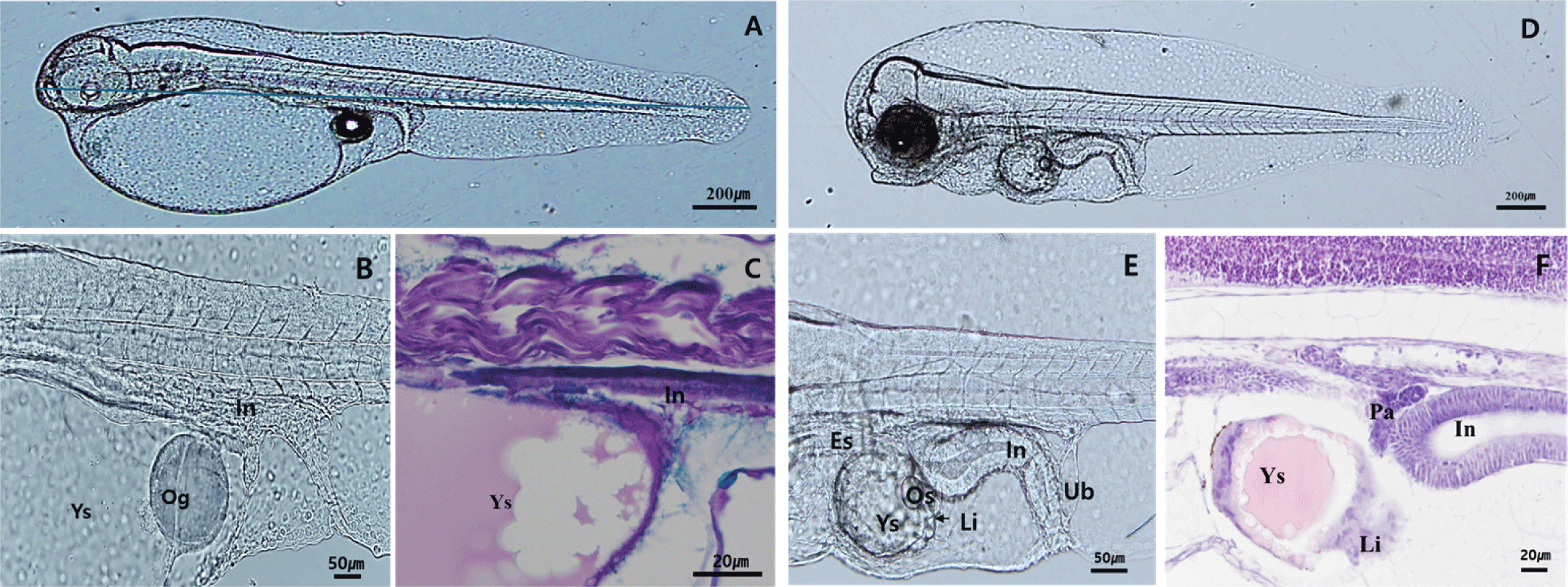
At 5 DAH, the larvae reached 2.36±0.17 mm TL, with more defined eye morphology due to increased melanophore deposition. The anus and mouth had opened, and rotifer ingestion was observed in the digestive tract (Fig. 2A). The digestive tract elongated and began to coil, showing clear differentiation between the intestine and rectum. The foregut and midgut were formed, and mucosal folds started to develop in the lumen (Fig. 2B and C). By 10 DAH, the larvae had grown to 2.92±0.23 mm TL, and the second dorsal fin and pelvic fin began to protrude (Fig. 2D). Teeth formation around the maxilla initiated, with increases in both size and number over time (Fig. 2E). Goblet cells were observed in the esophageal region, and the liver and pancreatic tissues showed further development. Mucosal folds in the rectum also began to appear, with goblet cells observed in the hindgut and rectum (Fig. 2F).
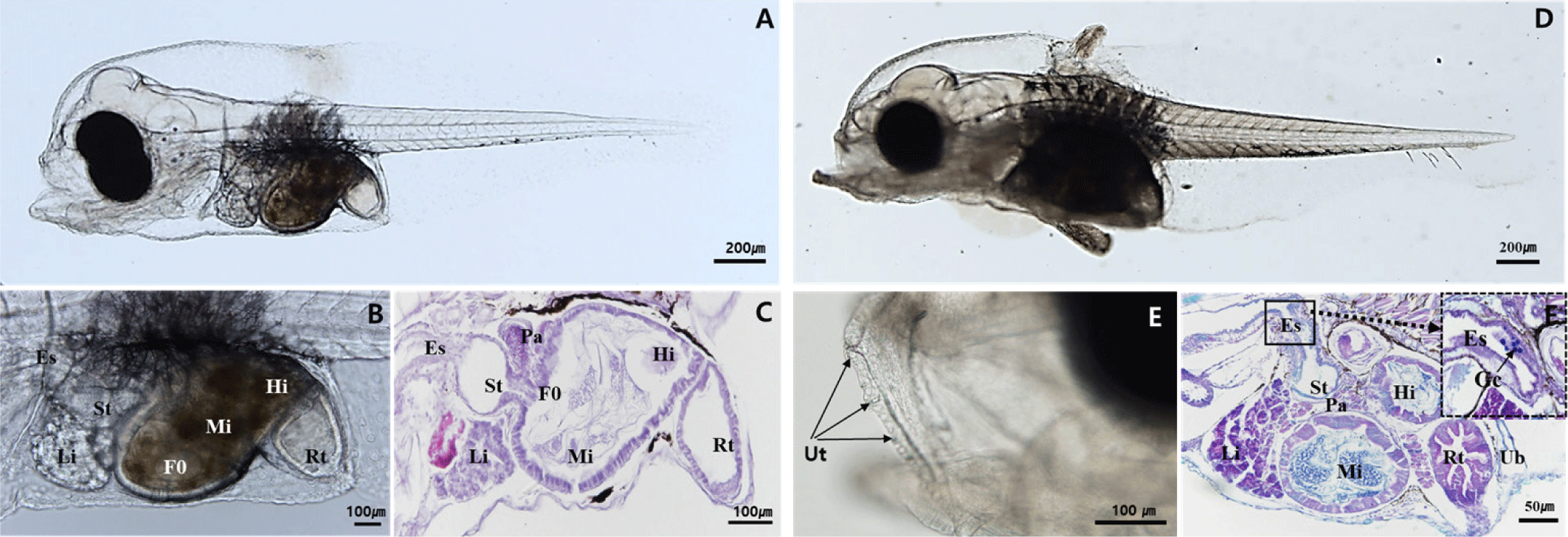
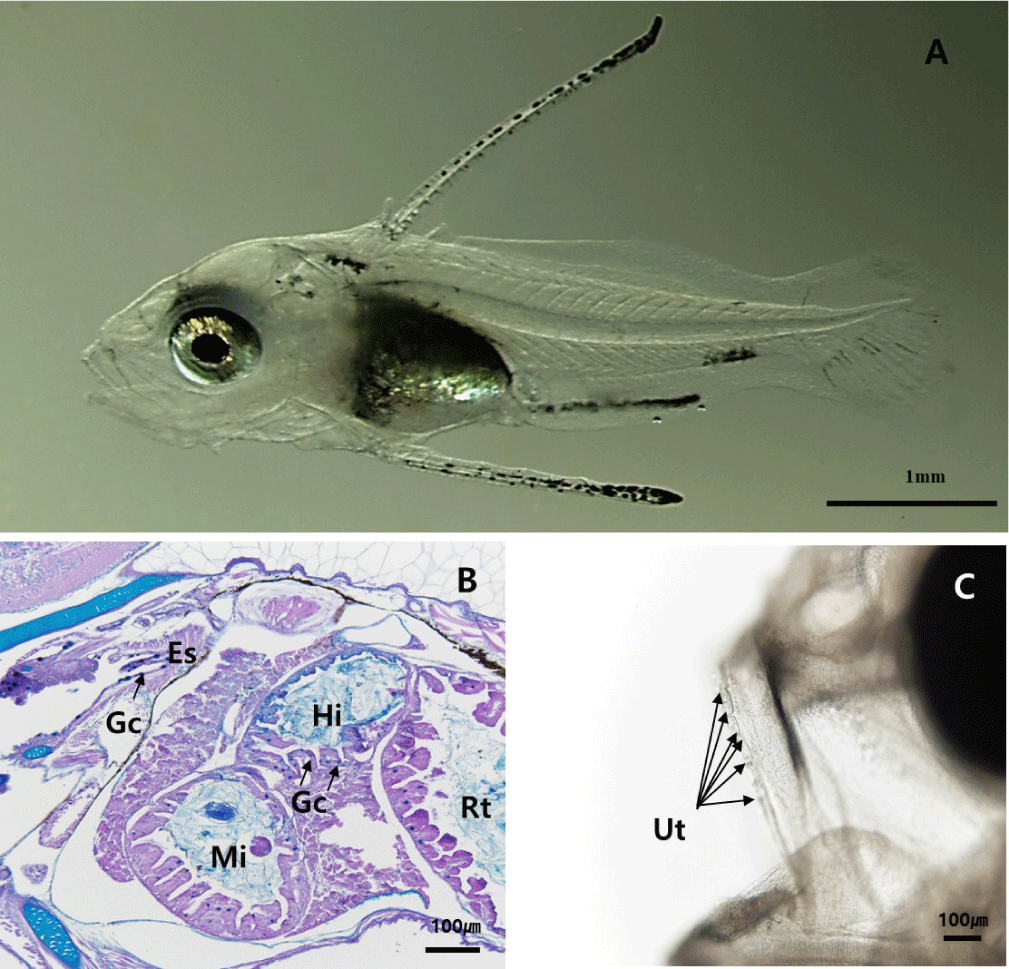
At 20 DAH, the larvae had grown to 6.09±0.80 mm TL. The first and third dorsal fins began to develop, and the second dorsal fin and pelvic fin continued to elongate (Fig. 3A). Alcian Blue-Periodic Acid Schiff (AB-PAS) staining revealed an increase in goblet cells within the esophagus and their presence throughout the digestive tract. Mucosal folds in the digestive tract continued to increase (Fig. 3B). The size and number of teeth increased as the larvae grew (Fig. 3C).
By 30 DAH, the larvae had reached 12.15±1.26 mm TL, with maximal elongation of the second dorsal fin and pelvic fin. Melanophore distribution expanded from the fin tips toward the body. Differentiation of the caudal fin progressed, characterized by the ossification of nine rays forming the upper and lower caudal fin lobes in the region of developing hypural plate (Fig. 4A). The pyloric caeca had formed, and mucosal folds in the stomach became more complex. Gastric glands were observed for the first time, indicating that the digestive system was developing towards adult-like morphology (Fig. 4B and C). Teeth were observed in the lower jaw and pharyngeal teeth (Fig. 4D). By 40 DAH, the larvae had grown to 13.43±1.59 mm TL, with some individuals beginning to develop pigmentation on the epidermis. Body height and width increased, and feeding responses became more active (Fig. 4E). The pyloric sphincter formed at the junction between the foregut and midgut, creating a clear boundary. The number of gastric glands increased, and the mucosal folds, as well as the overall digestive system, exhibited further complexity and development (Fig. 4F). Pharyngeal teeth continued to grow and develop (Fig. 4G).
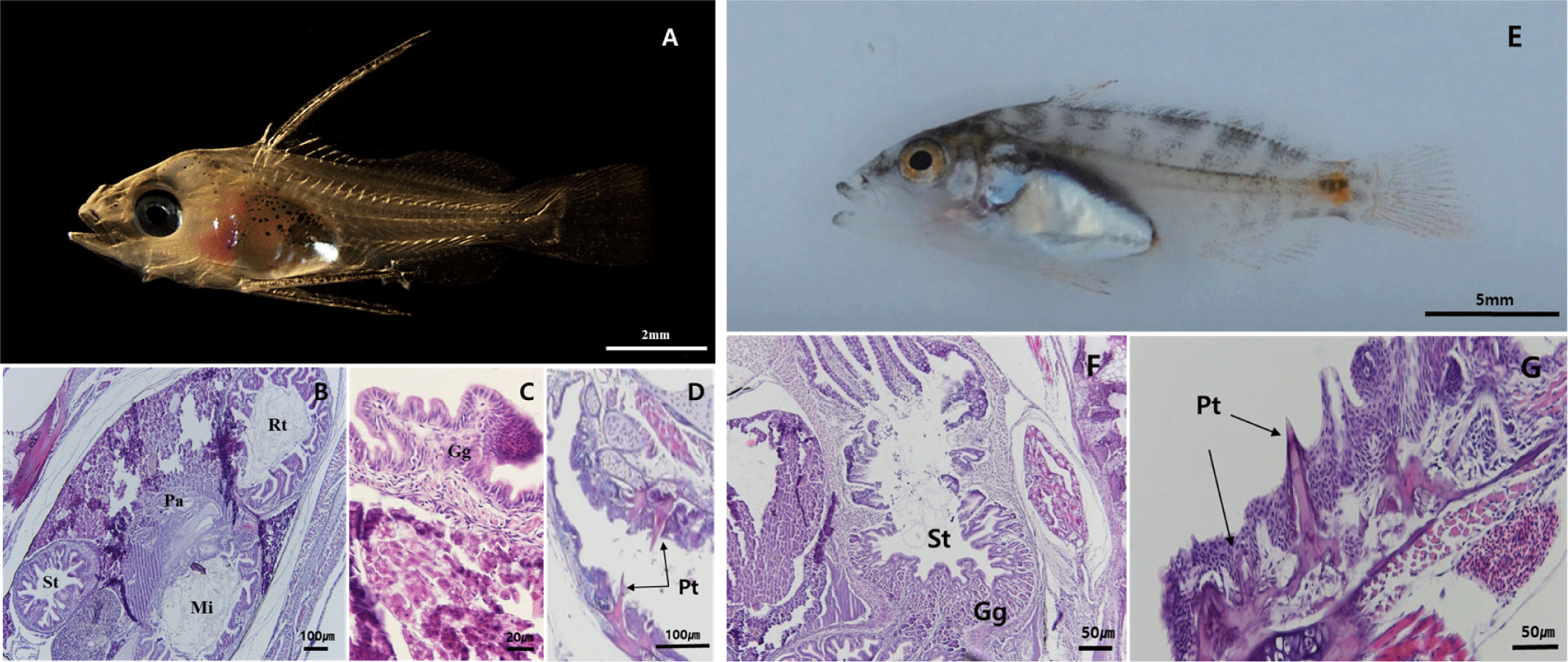
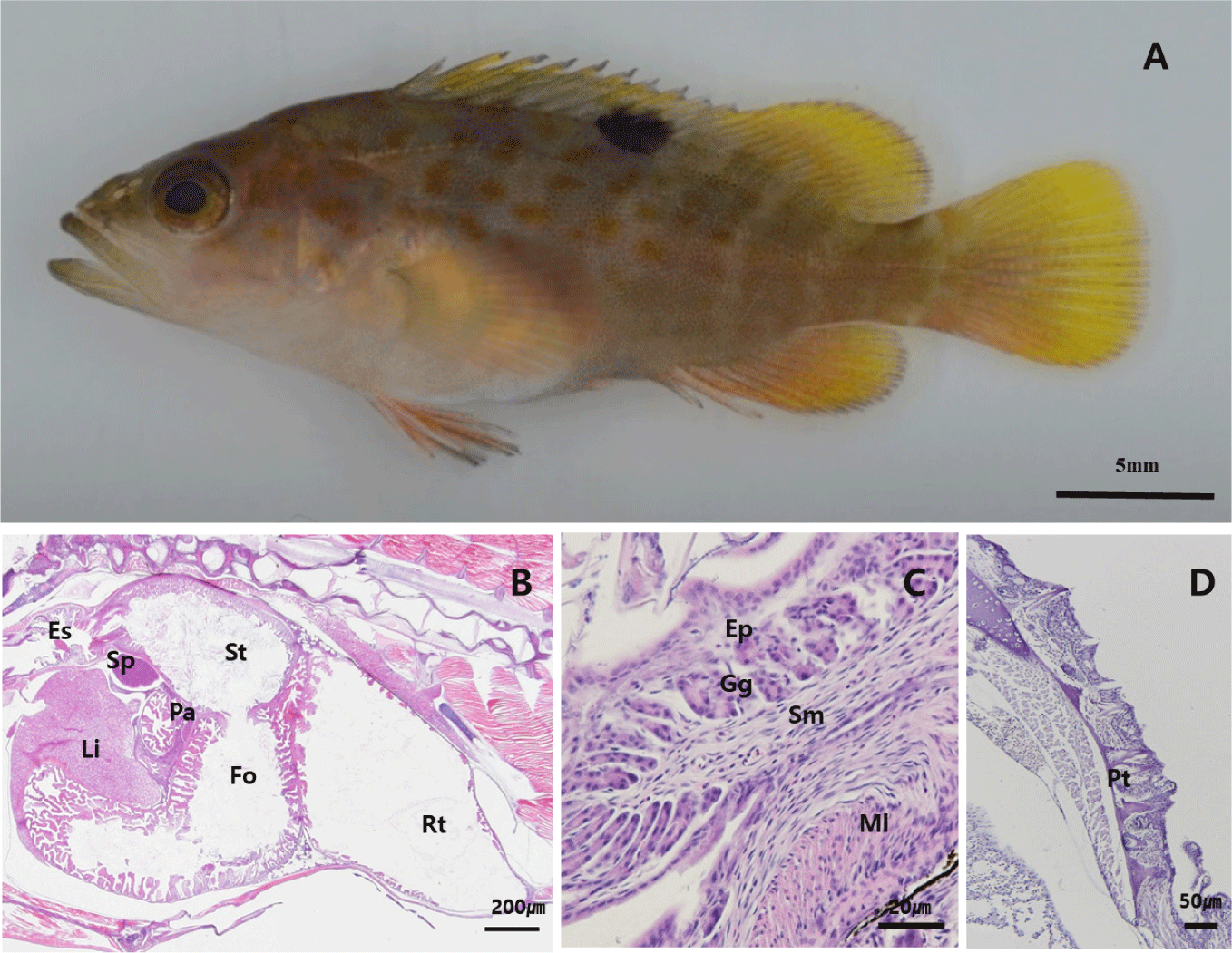
By 50 DAH, the juvenile had reached 23.8±6.0 mm TL, with fin and body coloration resembling that of adults (Fig. 5A). The gastric lumen had widened, and the development of gastric glands continued progressively. A dense distribution of mucosal folds was observed throughout the digestive tract (Fig. 5B). The pharyngeal teeth developed similarly to the adult fish (Fig. 5C).
DISCUSSION
The formation of digestive tract in fish involves two critical differentiation periods. The first occurs at the end of yolk-sac absorption, marking the onset of feeding. The second takes place during the transition from the larval to the juvenile stage, when gastric glands and pyloric caeca develop (Zambonino Infante et al., 2008; Nazemroaya et al., 2020). During yolk-sac absorption in red spotted grouper larvae (2–3 DAH), the rudimentary digestive system showed differentiation, and structures such as the esophagus, intestine, liver, and pancreas were observed. By 5 DAH, following the opening of the mouth and the completion of yolk-sac absorption, the intestinal lumen expanded rapidly, and the intestinal epithelium became distinct. Additionally, the liver and pancreas showed clear differentiation. The development of digestive tract during the endo-exotrophic stage in red spotted grouper larvae coincided with a previous study in other groupers, such as blacksaddled coralgrouper, Plectropomus laevis (Slamet & Melianawati, 2019), tiger grouper, Epinephelus fuscoguttatus (Aiemsomboon et al., 2017), leopard coral grouper, P. leopardus (Qu et al., 2012) and humpback grouper, Cromileptes altivelis (Abol-Munafi et al., 2011). As yolk absorption progresses, the differentiation and development of the liver and pancreas represent an essential adaptive process of the digestive organs, optimizing digestion and absorption at the onset of exogenous feeding (Martínez-Lagos et al., 2004; Qu et al., 2012).
The differentiation of gastric glands plays a key role in the development and function of the stomach. These glands enhance both the mechanical and enzymatic digestion of food, contributing to efficient nutrient processing. The presence of gastric glands is widely regarded as an indicator of a fully developed stomach.
Among most marine fish species, the development of a digestive system with a fully functional stomach and well-developed pyloric caeca signifies the transition to the juvenile stage (Nazemroaya et al., 2020). Histological observations revealed that the gastric glands and pyloric caeca in red spotted grouper larvae began to develop at 30 DAH and increased in number as the larvae grew. This indicates that gastric acid secretion and digestive enzyme activity are enhanced from day 30 onwards, marking a critical transition point from the late larval to juvenile stage. The timing of gastric gland and pyloric caeca formation varies among fish species, occurring at 20 DAH in turbot (Scophthalmus maximus) (Segner et al., 1994), 22 DAH in dover sole (Solea solea) (Boulhic & Gabaudan, 1992), 24 DAH in tiger grouper (E. fuscoguttatus) (Aiemsomboon et al., 2017), 25 DAH in humpback grouper (C. altivelis) (Abol-Munafi et al., 2011), and 90 DAH in Atlantic halibut (Hippoglossus hippoglossus) (Luizi et al., 1999). Some studies suggest that the initiation of diet weaning should coincide with the appearance of gastric glands and pyloric caeca (Chen et al., 2006; Qu et al., 2012). In Atlantic salmon (S. salar), the digestive system is fully developed around 46 DAH, allowing for nutritional supplementation through artificial feed (Sahlmann et al., 2015). For tiger grouper (E. fuscoguttatus), gastric glands and pyloric caeca are observed at 24 DAH, suggesting that diet switching should occur around this time (Aiemsomboon et al., 2017). This stage is considered optimal for transitioning to commercial pellets, which is directly associated with improved larval survival rates (Martínez-Lagos et al., 2004).
In conclusion, the development of digestive system in larval and juvenile red spotted grouper follows a pattern similar to that of other grouper species. During the early larval stage, the digestive system was functional enough to process exogenous food, but it reached maturity with the appearance of gastric glands and pyloric caeca at the onset of the late larval stage around 30 DAH. The development of gastric glands and pyloric caeca at this stage indicates that transitioning to commercial pellets is appropriate. Properly timing the diet transition is critical, as it directly affects larval survival and minimizes mass mortality. Therefore, determining the timing of gastric gland development through histological observation provides critical information for optimizing the timing of formulated diet introduction, contributing to productivity improvements in groupers, including the red spotted grouper.

